One of the most confusing ST-elevation mimics is the “strain pattern” (or repolarization abnormality) occasionally found with left ventricular hypertrophy.
This is important because left ventricular hypertrophy is one of the most common causes of ST segment elevation in chest pain patients.
Many 12 lead ECG classes teach to recognize the voltage criteria for LVH (or at least one of the voltage criteria) but I don’t think most 12 lead ECG classes do an adequate job explaining exactly what a “strain pattern” looks like.
As a result, once the student identifies the voltage criteria for LVH, the interpretation stops. Similarly, once the student identifies the presence of “wide” QRS complexes, the interpretation often stops.
It’s as if we’re teaching students that it’s impossible to identify STEMI in the presence of baseline abnormalities.
It’s more difficult, but it’s certainly not impossible. The whole point is to know what a “normal” abnormality looks like. This is not an oxymoron! It’s the key to advanced 12 lead ECG interpretation.
In many cases, an ECG can meet the voltage criteria for LVH but show only minimal distortion of the ST segments and T waves. In other cases, the ECG will show the characteristic ST segment depression and T wave inversion in the lateral leads, but not the exaggerated ST segment elevation and T wave prominence in the right precordial leads.
Let’s look at some examples. Let us assume that we are dealing with a patient complaining of chest discomfort.
 ECG courtesy of Dr. Jonas de Jong and ECGpedia.org
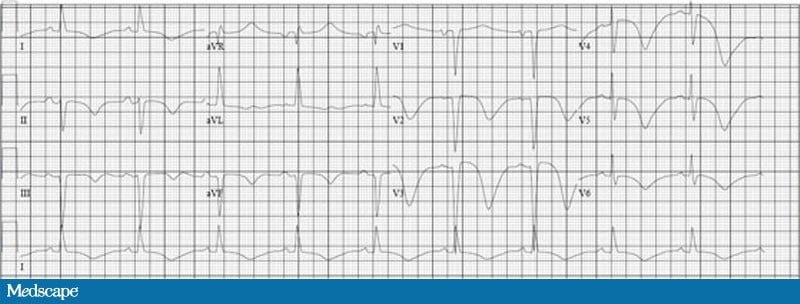
ECG courtesy of Dr. Jonas de Jong and ECGpedia.orgThis is exactly the kind of ECG that gives a lot of trouble. It demonstrates a strain pattern (or repolarization abnormality) with left ventricular hypertrophy. The good news is that it’s a very typical looking strain pattern!
Since this 12 lead ECG is not in the standard U.S. format, I used “cut” and “paste” to structure it into a pattern more typical of prehospital 12 lead ECGs in the U.S.

In the first place, you will notice that the rhythm is sinus at about 75 beats per minute (using the large block method).
The QRS width is less than 120 ms, so we know that we’re not dealing with a bundle branch block.
What about the ST segment elevation and huge T waves in the right precordial leads! Surely this patient is experiencing acute anterior STEMI!
Negative, ghostrider! (For my international friends, this is a reference to the movie Top Gun).
Let’s look at the relationship between the QRS complex and the T waves in this ECG. The general pattern is one of discordance. In other words, When the QRS complex is positive (especially in the lateral leads I, aVL, V5 and V6) the T wave is negative. This is sometimes referred to as a widened QRS/T angle.
In addition, the ST segments are downwardly concave and the T waves are asymmetrical.
These are the cardinal findings with strain patterns (or repolarization abnormalities) secondary to left ventricular hypertrophy.
This ECG also shows ST segment elevation in the right precordial leads (V1, V2 and V3). You will note that the ST segments are upwardly concave and the severity of the ST segment elevation and T wave height is proportional to the depth of the S wave.
This is extremely important! With left ventricular hypertrophy, the deeper the QRS complex, the higher the ST segment and more pronounced the T wave abnormality.
This is also true of the ST segment depression and T wave inversion typically found in the lateral leads. The higher the R wave, the deeper the ST segment depression and more pronounced the inverted T wave.
Consider the following graphics to illustrate the point.
The most pronounced ST/T wave abnormality is found in lead V2. It’s difficult to tell because the QRS complexes run into one another, but the S wave is extremely deep in lead V2, possibly as deep as 35 mm (blue arrows). With LVH, you should expect the lead with the deepest S wave to show the most ST segment elevation and/or T wave height!
The red curve shows the upward concavity of the ST segment, which is another common finding with LVH. I have seen upwardly convex ST segments with LVH, but it’s rare, and it always makes me suspicious of acute anterior STEMI!
I’ve outlined the shape of the T wave with orange lines. You can see that the T waves are asymmetrical, another finding consistent with a “strain pattern” or depolarization abnormality with LVH.
In the left precordial leads, the most most pronounced ST/T wave abnormality is found in lead V5. Again, it’s difficult to discern because the QRS complexes run into one another (as they often do with LVH) but the height of the R wave may be as high as 30 or even 40 mm (blue arrows).
The red curve shows the downwardly concave ST segment depression (exactly opposite the right precordial leads).
I have outlined the T wave inversion with orange lines to show the asymmetry. Again, a common finding with “strain patterns” or depolarization abnormalities with LVH.
Example #1.
This is an atypical “strain pattern” with many typical features.
I suspect the possibility that leads V1 and V2 might have been accidentally transposed but that doesn’t really matter. For the purposes of STEMI recognition, the typical features outweigh the atypical features.
In the first place, you should immediately notice the “widened QRS-T angle” that is the hallmark of a secondary repolarization abnormality. You will notice the same finding for LBBB and paced rhythm!
Importantly, the degree of the secondary ST-T abnormality is, generally speaking (there are some caveats), proportional to the size (or amplitude) of the QRS complex in the opposite direction!
If you take nothing else away from this post, please learn this “trick”.
Herein lies a problem with prehospital 12-lead ECGs!
With left ventricular hypertrophy (LVH) the QRS complexes are often “cut off” at the top or bottom or they run together with other QRS complexes which can create the illusion that the QRS complexes are smaller, so you have to train your eye!
Take a look at this ECG and find the most severe ST-T wave abnormality.
That’s easy! Lead I clearly shows the most pronounced ST-T wave abnormality. The ST-segment is depressed, downwardly concave, and shows a deep inverted T-wave.
Does the amplitude of the R-wave in the opposite direction explain it? No way! It’s even smaller than the QRS complex in lead II, and the ST-T wave abnormality in lead II isn’t nearly as severe!
What is the second-worse ST-T wave abnormality? Lead V3! Does the depth of the S-wave in the opposite direction explain it? Not really.
But wait! Are we certain we’re getting an accurate “read” on the amplitude of the R-wave in lead I and the depth of the S-wave in lead V3?
I’m not so sure!
I suspect the possibility that the computer is “cropping” the QRS complexes to fit them on the ECG paper. See the little horizontal line that marks the “top” and “bottom” of these QRS complexes?
I’ve seen it many times before!
So ask yourself this question:
Generally speaking, does there seem to be a relationship between the QRS-complex and the degree of ST-elevation or depression in the opposite direction?
If the answer is “Yes!” then don’t call the STEMI Alert. Instead, perform serial ECGs and look for changing ST-segments and T-waves! ST-segments and T-waves shouldn’t “evolve” or change over time if it’s a simple secondary ST-T wave abnormality!
Example #2